 Clomid for Men With Low
Clomid for Men With Low Testosterone Part Four
This article is part four of a series. Part One, Part two, Part Three
Infertility is a major adverse effect of testosterone therapy in young males. For this reason, testosterone therapy is usually avoided in this group. A better solution is clomiphene, FDA approved as a a fertility drug for women. Although not specifically FDA approved for use in males, it is frequently used "off-label" and is preferable to testosterone therapy, since clomiphene will raise testosterone levels without the adverse effect of infertility.
New studies over 2013-2014 published in the urology and endocrinology literature show renewed interest in Clomiphene treatment to increase testosterone levels in hypogonadal males. These recent studies find clomiphene just as effective as testosterone therapy itself, without the adverse side effect of infertility produced by testosterone treatment.(1-9)
 The
studies find that Clomid (Clomiphene) actually increases FSH levels,
which stimulates increased sperm counts and preserves fertility. This
is important in the younger male age group who wish to preserve
fertility.(1-9)
The
studies find that Clomid (Clomiphene) actually increases FSH levels,
which stimulates increased sperm counts and preserves fertility. This
is important in the younger male age group who wish to preserve
fertility.(1-9)Addition of Aromatase Inhibitor
Dr. Johnson found that 16% of males treated with clomiphene developed elevated estradiol levels, and were prescribed an aromatase inhibitor, anastrazole. This group of men tended to be obese.
Recent studies on clomiphene to raise testosterone: Click Here.
Repros Therapeutics has a patent on enclomiphene citrate, trade name Androxal, and has been funding controlled trials.
link for more enclomiphene studies. This article is part four of a series. Click here for Part One, Part two, Part Three.
Left Images courtesy of Wikimedia commons (10-11)
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Florida 33314
954-792-4663
Links and References
1) http://www.foxnews.com/health/
New drug could treat low testosterone with fewer side effects
LiveScience By Christopher Wanjek
2) http://patch.com/california/
The Best Testosterone Booster for Men with Low Testosterone Levels. Dr. Shira Miller
3) http://shiramillermd.com/blog/
Clomiphene Citrate (Clomid) - A Testosterone Therapy Alternative for Men with Low Testosterone Levels Dr. Shira Miller
4) http://peaktestosterone.com/
Clomid and Testosterone
---------------------------
5) http://press.endocrine.org/
Endocrine Society's 96th Annual Meeting and Expo, June 21–24, 2014 - Chicago
Clomiphene Citrate in Anabolic Steroid Users: A Retrospective Review Mirjana Pavlic1 and Richard Arthur Bebb2
1University of British Columbia, Vancouver, Canada
2Univ of British Columbia, Vancouver, Canada
Date of Presentation: June, 2014
This is the first larger retrospective case series to demonstrate clear benefit of clomiphene in restoring HPG axis in AAS users.
6) http://www.ncbi.nlm.nih.gov/
J Sex Med. 2013 Jun;10(6):1628-35. doi: 10.1111/jsm.12116. Epub 2013 Mar 26.
Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel.
Kaminetsky J1, Werner M, Fontenot G, Wiehle RD.
AIM:Our aim was to compare oral enclomiphene citrate as an alternative to topical testosterone.
MAIN OUTCOME MEASURES:Blood levels of total testosterone (TT), estradiol, follicle-stimulating hormone (FSH), luteinizing hormone (LH), sex hormone binding globulin, thyroid stimulation hormone, prolactin, and insulin-like growth factor 1 IGF-1 were measured at certain times after treatment with each agent. Sperm parameters were determined at the same visits. Free testosterone (FT) was calculated.
METHODS:This was a proof-of-principle, randomized, open-label, fixed dose, active-control, two-center phase IIB study in 12 men with secondary hypogonadism treated previously with topical testosterone.
RESULTS:After discontinuation of topical testosterone, morning TT values averaged 165 ± 66 pg/dL. After 3 months, there was a significant rise in men receiving enclomiphene citrate and gel that was sustained for 3 months. At 6 months, TT levels were 545 ± 268 and 525 ± 256 pg/dL for groups receiving the gel and enclomiphene citrate, respectively. Only men in the enclomiphene citrate group demonstrated increased LH and FSH. TT decreased one month posttreatment to pretreatment values. Enclomiphene citrate elevated sperm counts in seven out of seven men at 3 months and six out of six men at 6 months with sperm concentrations in the 75-334 × 10(6) /mL range. The gel was ineffective in raising sperm counts above 20 × 10(6) /mL for all five men at 3 months and raised counts in only two or five men at 6 months. At follow-up, only enclomiphene citrate treatment was associated with elevated sperm counts.
CONCLUSIONS:Enclomiphene citrate increased testosterone and sperm counts. Concomitant changes in LH and FSH suggest normalization of endogenous testosterone production and restoration of sperm counts through the hypothalamic-pituitary-
7) http://www.ncbi.nlm.nih.gov/
J Urol. 2014 Sep;192(3):875-9. doi: 10.1016/j.juro.2014.03.089. Epub 2014 Mar 21. Testosterone supplementation versus clomiphene citrate for hypogonadism: an age matched comparison of satisfaction and efficacy.
Ramasamy R1, Scovell JM1, Kovac JR1, Lipshultz LI2.
We compared satisfaction and treatment efficacy in men with symptomatic hypogonadism who received clomiphene citrate or testosterone supplementation therapy.
MATERIALS AND METHODS: Men treated with clomiphene citrate, or testosterone injections or gels for symptomatic hypogonadism (total testosterone less than 300 ng/dl) reported satisfaction with the current treatment regimen using the qADAM questionnaire.
RESULTS: A total of 93 men on testosterone injections (31) or gels (31), or clomiphene citrate (31) were age matched from a retrospective cohort of 1,150 on testosterone supplementation therapy. We compared men who received testosterone supplementation therapy to 31 not on such therapy, who served as controls. Median serum testosterone increased from pretreatment levels in all men regardless of therapy with clomiphene citrate, and testosterone injections and gels (from 247 to 504, 224 to 1,104 and 230 to 412 ng/dl, respectively, p <0.05). Final median serum total testosterone in men on clomiphene citrate (504 ng/dl) was lower than in men receiving testosterone injections (1,014 ng/dl, p <0.01) but similar to that in men on testosterone gels (412 ng/dl, p = 0.31). Despite different serum testosterone levels men on all 3 therapies reported similar satisfaction on qADAM, including a score of 35 for clomiphene citrate, 39 for testosterone injections, 36 for testosterone gels and 34 for control treatment (p >0.05). Men receiving testosterone injections reported greater libido than men on clomiphene citrate (4 vs 3, p = 0.04) or testosterone gels (4 vs 3, p = 0.04), or controls (4 vs 3, p <0.01).
CONCLUSIONS: Testosterone supplementation regimens and clomiphene citrate are efficacious for improving serum total testosterone. No difference in overall hypogonadal symptoms was noted among men on any testosterone supplementation therapy. Despite lower serum total testosterone, men on clomiphene citrate and testosterone gels reported satisfaction similar to that of men treated with testosterone injections.
8) http://www.ncbi.nlm.nih.gov/
BJU Int. 2013 Jul 12. doi: 10.1111/bju.12363. [Epub ahead of print]
Testosterone Restoration by Enclomiphene Citrate in Men with Secondary Hypogonadism: Pharmacodynamics and Pharmacokinetics.
Wiehle R1, Cunningham GR, Pitteloud N, Wike J, Hsu K, Fontenot GK, Rosner M, Dwyer A, Podolski J.
To determine the pharmacodynamic (PD) profile of serum total testosterone levels (TT) and luteinizing hormone (LH) in men with secondary hypogonadism following initial and chronic daily oral doses of enclomiphene citrate in comparison to transdermal testosterone. To determine the effects of daily oral doses of enclomiphene citrate (Androxal®) in comparison to transdermal testosterone on other hormones and markers in men with secondary hypogonadism.
PATIENTS AND METHODS:This was a randomized, single blind, two-center phase II study to evaluate three different doses of enclomiphene citrate (6.25mg, 12.5mg and 25 mg Androxal®), versus AndroGel®, a transdermal testosterone, on 24-hour LH and TT in otherwise normal healthy men with secondary hypogonadism. Forty-eight men were enrolled in the trial (ITT Population), but 4 men had T levels >350 ng/dL at baseline. Forty-four men completed the study per protocol (PP population). All subjects enrolled in this trial had serum TT in the low range (<350 ng/dL) and had low to normal LH (<12 IU/L) on at least two occasions. TT and LH levels were assessed each hour for 24 hours to examine the effects at each of three treatment doses of enclomiphene versus a standard dose (5 grams) of transdermal testosterone (AndroGel). In the initial profile TT and LH were determined in a naïve population following a single initial oral or transdermal treatment (Day 1). This was contrasted to that seen after six weeks of continuous daily oral or transdermal treatment (Day 42). The pharmacokinetics of enclomiphene was performed in a select subpopulation. Serum samples were obtained over the course of the study to determine levels of various hormones and lipids.
RESULTS:After six weeks of continuous use, the mean ± SD concentration of TT at Day 42 C0hrTT, was 604 ± 160 ng/dL for men taking the highest of dose of enclomiphene citrate (enclomiphene, 25 mg daily) and 500 ± 278 ng in those men treated with transdermal testosterone. These values were higher than Day 1 values but not different from each other (p = 0.23, T-test). All three doses of enclomiphene increased C0hrTT, CavgTT, CmaxTT, CminTT and CrangeTT. Transdermal testosterone also raised TT, albeit with more variability, and with suppressed LH levels. The patterns of TT over 24 hour period following six weeks of dosing could be fit to a non-linear function with morning elevations, mid-day troughs, and rising night-time levels. Enclomiphene and transdermal testosterone increased levels of TT within two weeks, but they had opposite effects on FSH and LH Treatment with enclomiphene did not significantly affect levels of TSH, ACTH, cortisol, lipids, or bone markers. Both transdermal testosterone and enclomiphene citrate decreased IGF-1 levels (p<0.05) but suppression was greater in the enclomiphene citrate groups.
CONCLUSIONS:Enclomiphene citrate increased serum LH and TT; however, there was not a temporal association between the peak drug levels and the Cmax levels LH or TT. Enclomiphene citrate consistently increased serum TT into the normal range and increased LH and FSH above the normal range. The effects on LH and TT persisted for at least one week after stopping treatment.
9) http://www.ncbi.nlm.nih.gov/
J Sex Med. 2014 Sep;11(9):2302-7. doi: 10.1111/jsm.12592. Epub 2014 Jun 5.
Predicting biochemical response to clomiphene citrate in men with hypogonadism.
Mazzola CR1, Katz DJ, Loghmanieh N, Nelson CJ, Mulhall JP.
Clomiphene citrate (CC) is as an effective treatment for men with hypogonadism (HG). Identifying the ideal candidate for this strategy has to date largely relied upon a patient's interest in preservation of testicular volume and spermatogenesis.
AIM:This analysis was undertaken to define if predictors existed of robust elevation in serum testosterone (T) levels in response to CC.
METHODS:Seventy-six men with a diagnosis of HG (two separate early morning total T levels <300 ng/dL) opting for CC therapy constituted the study population. Demographic, comorbidity data, and physical and laboratory characteristics were recorded. Laboratory tests were conducted 4 weeks after commencement and every 6 months thereafter. Multivariable analysis was conducted to define if predictors of biochemical response could be identified. Parameters included in the model were patient age, mean testicular volume, varicocele presence, and baseline total T, free T, and luteinizing hormone (LH) levels.
MAIN OUTCOME MEASURE:Successful biochemical response to CC, defined as an increase of ≥200 ng/dL in total T level at ≥6 months after commencing CC, was the main outcome measure.
RESULTS:Mean age was 46 ± 22 years. Mean pretreatment testicular volume was 16 ± 8 mL. Mean baseline T and LH levels were 179 ± 72 ng/dL and 7.2 ± 5.6 IU/mL, respectively. Mean total T on CC was 467 ± 190 ng/dL. Forty-seven patients (62%) met the responder definition, with a mean increase in total T levels of 302 ± 76 (204-464) ng/dL. In CC responders, the mean LH rise was 5.6 ± 3.1 IU/mL. On multivariable analysis, factors predictive of CC response included: mean testicular volume (adjusted [adj.] r = 0.32, P < 0.01), mean testicular volume ≥14 mL (hazard ratio [HR] 2.2, P < 0.01), LH level (adj. r = 0.48, P < 0.001), and LH level ≤6 IU/mL (HR 3.5, P < 0.001).
CONCLUSION:These data indicate that two thirds of men with HG meet a robust responder definition and that pretreatment testicular volume and LH levels (in continuous and dichotomized fashions) are predictors of response. Mazzola CR, Katz DJ, Loghmanieh N, Nelson CJ, and Mulhall JP. Predicting biochemical response to clomiphene citrate in men with hypogonadism. J Sex Med 2014;11:2302-2307.
Images
10) http://commons.wikimedia.org/wiki/File:US_Navy_070504-N-0995C-072_Chief_Mineman_Kevin_Sperling_appears_as_the_guest_body_builder_ARL HARBOR, Hawaii (May 4, 2007) - Chief Mineman Kevin Sperling appears as the guest body builder at an Armed Forces body building competition held at Sharkey's Theatre at Naval Station Pearl Harbor. Sperling is an assistant 3-M coordinator aboard guided-missile destroyer USS Hopper (DDG 70). U.S. Navy photo by Mass Communication Specialist Seaman Eric J. Cutright (RELEASED) Date 4 May 2007
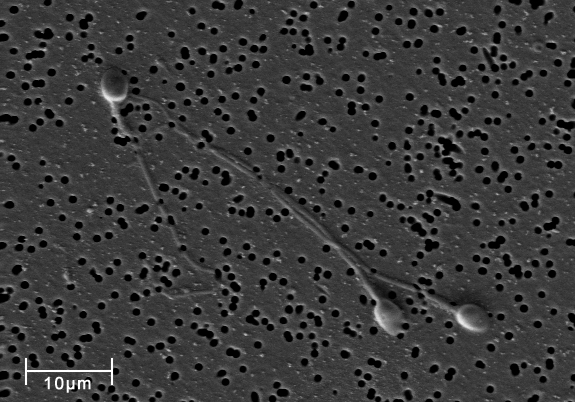
11) http://commons.wikimedia.org/wiki/File:Spermatozoa-human-3140x.jpg
English: Micrograph by scanning electron microscope (SEM) of human sperm . Date 14 October 2010
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Florida 33314
954-792-4663
http://www.jeffreydachmd.com
http://www.drdach.com/
http://www.naturalmedicine101.com/
http://www.truemedmd.com/
http://www.bioidenticalhormones101.com/
For Disclaimer: Click Here
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship.
Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article
Copyright (c) 2014 Jeffrey Dach MD All Rights Reserved
This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation
No comments:
Post a Comment