This information was published originally in 2002 and again in 2011 in JAMA by the same authors. See my previous article which discussed this.
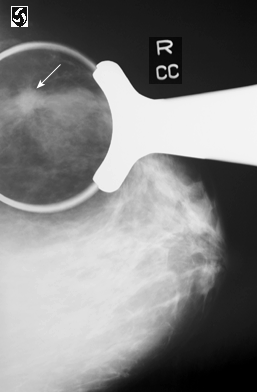
Above image: white arrow points to breast cancer, courtesy of NIH and Wikimedia commons
Prempro Causes Breast Cancer and Heart Disease
The NIH sponsored Women's Health Initiative study was terminated early because Prempro, was found to cause breast cancer and heart disease. Prempro contains Provera (medroxyprogesteone), and Premarin, estrogen from pregnant horses. Provera is a synthetic chemically altered progesterone never found in nature or in the human body, and for years has been known to cause cancer and heart disease, much like other chemically altered hormones. These chemically altered hormones are, in fact, monsters which should never have been approved for human consumption.
More Breast Cancer in the Prempro Users
Over 11 years of observation, the (Prempro) estrogen plus progestin users had 0.60% incidence of breast cancer, while the non-users had 0.42%. This is roughly one and a half times more breast cancer in the Prempro users.
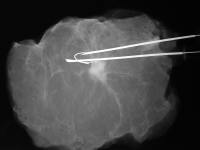
Left image: breast cancer in specimen radiograph with Homer needle in place, used to localize the lesion. Coutesy of Jeffrey Dach MD personal archives.
How Do We Know Provera (medroxyprogestroene) Caused the Increased Breast Cancer?
We know this because the NIH study included another group that was given Prempro minus the Provera. These women had hysterectomies, so did not need the progestin, Provera. They were given solely Premarin, the horse estrogen. These Premarin treated women actually had reduced incidence of breast cancer compared to controls, 23% less, as matter of fact. This is discussed in my previous article, here.
How to Prevent Breast Cancer
1) Avoid Prempro- and all other synthetic hormone pills, xeno-estrogens, pesticides and chemicals that increase breast cancer rates.
2) Use Bio-Identical Hormones-these are naturally found in the human body and do not increase breast cancer rates or heart disease.
3) Use Iodine, Vitamin D and Selenium supplementation.
Jeffrey Dach MD
Articles with related interest:
The Safety of Bioidentical Hormones
Bioidentical Hormone Videos - Radio Show- Jeffrey Dach MD
Bioidentical Hormones and Medical GhostWriting by Jeffrey Dach MD
Bioidentical Hormones Prevent and Reverse Osteoarthritis by Jeffrey Dach
The Importance of BioIdentical Hormones
Bioidentical Hormones, How to Wake Up From the Synthetic Hormone Nightmare
Are Bio-Identical Hormones Safe?
Bioidentical Hormones Reverse Aging New Harvard Study by Jeffrey Dach MD
Why Bioidentical Hormones Are Better Than Synthetic by Jeffrey Dach MD
BioIdentical Hormones Relieve Anxiety by Jeffrey Dach MD
Bioidentical Hormones Found Beneficial After Hysterectomy
Menopausal Arthritis and Bioidentical Hormones by Jeffrey Dach MD
The Evidence for Bioidentical Hormones by Jeffrey Dach MD
The Importance of BioIdentical Hormones by Jeffrey Dach MD
Links and References:
1) http://jnci.oxfordjournals.org/content/early/2013/03/29/jnci.djt092.full
Estrogen Plus Progestin and Breast Cancer Incidence and Mortality in the Women’s Health Initiative Observational Study by Rowan T. Chlebowski, JoAnn E. Manson, published March 29 in the Journal of the National Cancer Institute. Affiliation of authors: Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA (RTC); Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (JEM); Fred Hutchinson Cancer Research Center, Seattle, WA (GLA, CC, AKA, PAN); University of Pittsburgh, Pittsburgh, PA (JAC); Stanford University, Stanford, CA (MLS); State University of New York, Stony Brook, NY (DSL); University of Tennessee Health Science Center, Memphis, TN (KCJ); University at Buffalo, Buffalo, NY (JWW); University of California at Davis, Sacramento, CA (LQ, SY).
Background In the Women’s Health Initiative (WHI) randomized trial, estrogen plus progestin increased both breast cancer incidence and mortality. In contrast, most observational studies associate estrogen plus progestin with favorable prognosis breast cancers. To address differences, a cohort of WHI observational study participants with characteristics similar to the WHI clinical trial was studied.
Methods We identified 41, 449 postmenopausal women with no prior hysterectomy and mammogram negative within 2 years who were either not hormone users (n = 25 328) or estrogen and progestin users (n = 16 121). Multivariable-adjusted Cox proportional hazard regression was used to calculate hazard ratios (HRs) with 95% confidence intervals (CI). All statistical tests were two-sided.
Results After a mean of 11.3 (SD = 3.1) years, with 2236 breast cancers, incidence was higher in estrogen plus progestin users than in nonusers (0.60% vs 0.42%, annualized rate, respectively; HR = 1.55, 95% CI = 1.41 to 1.70, P < .001). Women initiating hormone therapy closer to menopause had higher breast cancer risk with linear diminishing influence as time from menopause increased (P < .001). Survival after breast cancer, measured from diagnosis, was similar in combined hormone therapy users and nonusers (HR = 1.03, 95% CI = 0.79 to 1.35). On a population basis, there were somewhat more deaths from breast cancer, measured from cohort entry (HR = 1.32, 95% CI = 0.90 to 1.93, P = .15), and more all-cause deaths after breast cancer (HR = 1.65, 95% CI = 1.29 to 2.12, P < .001) in estrogen plus progestin users than in nonusers.
Conclusions Consistent with WHI randomized trial findings, estrogen plus progestin use is associated with increased breast cancer incidence. Because prognosis after diagnosis on combined hormone therapy is similar to that of nonusers, increased breast cancer mortality can be expected.
2) http://www.myhealthnewsdaily.com/3678-hrt-breast-cancer-risk.html
Hormone Therapy May Raise Risk of Aggressive Breast Cancers
Mar 29, 2013 | 4:13 PM ET | Rachael Rettner, MyHealthNewsDaily Staff Writer
Women who undergo hormone-replacement therapy (HRT) to treat symptoms of menopause are at increased risk of developing all categories of breast cancer, a new study has found. In the study, postmenopausal women on hormone replacement therapy that included both estrogen and progestin were 1.5 times more likely to develop breast cancer over an 11-year period compared with women not on the hormones. HRT increased the risk of breast cancers that have a low risk of recurrence, such as estrogen-receptor-positive cancers, as well as the risk of more aggressive breast cancers, such as triple-negative breast cancer. The findings back up the results of a study published last year that suggested HRT increased the risk of all categories of breast cancer. Before that study, doctors thought that HRT only increased the risk of less-serious cancers, said study researcher Dr. Rowan Chlebowski, of the Los Angeles Biomedical Research Institute. The new findings lead to more of a consensus on the link between HRT and breast cancer, and suggest doctors should exercise even more caution when prescribing the treatment, Chlebowski said.
The decision to start HRT should be made on a case-by-case basis, Chlebowski said. Women should speak with their doctors about the risks and benefits of the therapy. The benefits will be greater for those with more severe symptoms of menopause, Chlebowski said. The National Institutes of Health has stated that if women decide to receive HRT, they should take the lowest dose for the shortest amount of time, and be re-evaluated every six months to see if they still need the treatment. Recently, several doctors groups said that for women under age 60, or for those who reached menopause within the past 10 years, the benefits of HRT generally outweigh the risks. The link between HRT use and an increased risk of breast cancer was first seen in 2002, when a large study on the effects of estrogen and progestin therapy was suspended because researchers found the treatment increased the risk of invasive breast cancer.. The new study analyzed information from 41,000 postmenopausal women ages 50 to 79, about half of whom were taking HRT (estrogen plus progestin) at some point in the study, and half who were not on the therapy. During the study period, about 2,200 women were diagnosed with invasive breast cancer. Among those who took HRT, 0.6 percent developed breast cancer each year, compared with 0.42 percent for those who weren’t on HRT. Women's survival after a breast-cancer diagnosis was about the same for both groups. However, because more women who took HRT developed breast cancer, the findings suggest that the use of HRT may increase deaths from breast cancer in the population as a whole, the researchers said. The findings support what doctors have already been doing to recognize the risk of breast cancer with HRT, said Dr. Erin Olson, an oncologist at Ohio State' s James Cancer Hospital who was not involved in the study. In an editorial accompanying the study, Catherine Schairer and Louise Brinton, of the National Cancer Institute, said that the new study does not provide definitive evidence that HRT increases the risk of both low-risk and aggressive cancers. To answer this question, more studies will need to take into account the length of time the women are on the therapy. The study was published in the March 29 issue of the Journal of the National Cancer Institute.
3) Phyllis J. Bronson, Ph.D.
Biochemical Consulting Company
Biochemical Research Foundation, Aspen
University of Denver: Department of Chemistry/Biochemistry
International Society for Orthomolecular Medicine
Biochemical Consulting Company
Biochemical Research Foundation, Aspen
University of Denver: Department of Chemistry/Biochemistry
International Society for Orthomolecular Medicine
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydach.com
www.drdach.com
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
www.bioidenticalmds.com
Click Here for: Dr Dach's Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach's Online Store for Nature's Sunshine Supplements
Web Sites and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244124.html
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
disc.yourwebapps.com/Indices/244161.html
disc.yourwebapps.com/Indices/244163.html
disc.yourwebapps.com/Indices/244163.html
health-forums.1
health-forums.2
Disclaimer click here: www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician -- patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article:http://wp.me/p3gFbV-9S
Copyright (c) 2013 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a 'fair use' of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
No comments:
Post a Comment